
Chemistry, 16.08.2020 14:01 animaljamissofab
In the following net ionic equation, identify each reactant as either a Bronsted-Lowry acid or a Bronsted-Lowry base. HCN(aq) H2O(l) CN-(aq) H3O (aq) B-L B-L
The formula of the reactant that acts as a proton donor is
The formula of the reactant that acts as a proton acceptor is

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1


Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3
You know the right answer?
In the following net ionic equation, identify each reactant as either a Bronsted-Lowry acid or a Bro...
Questions


Mathematics, 01.07.2021 17:30



Mathematics, 01.07.2021 17:30








Computers and Technology, 01.07.2021 17:30

Social Studies, 01.07.2021 17:30



Social Studies, 01.07.2021 17:30



Social Studies, 01.07.2021 17:30

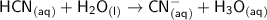
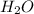 accepts the H⁺ ion ,thus it is a Bronsted-Lowry base.
accepts the H⁺ ion ,thus it is a Bronsted-Lowry base.

