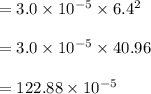Chemistry, 17.08.2020 01:01 nicholasferrell
The reaction NO2(g) + CO(g) → NO(g) + CO2(g) has been found to be second order with respect to NO2 and zero order with respect to CO. At a certain temperature, the rate constant is found experimentally to be 3.0 × 10−5 L mol · s . What is the rate of formation

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
**40** points asapessay questions (10 points possible) clear image of next, create your own scenario. it can be one of your own real experiences or one you make up. use imagery in your writing to give your instructor a the setting and an action taking pace in your writing explain the structure and functions of the skin at work in your scenario. !
Answers: 3

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
The reaction NO2(g) + CO(g) → NO(g) + CO2(g) has been found to be second order with respect to NO2 a...
Questions



Mathematics, 28.04.2021 20:10

Mathematics, 28.04.2021 20:10

Mathematics, 28.04.2021 20:10



History, 28.04.2021 20:10

Mathematics, 28.04.2021 20:10


Chemistry, 28.04.2021 20:10

Mathematics, 28.04.2021 20:10



English, 28.04.2021 20:10

Mathematics, 28.04.2021 20:10



Arts, 28.04.2021 20:10

History, 28.04.2021 20:10

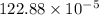 "
"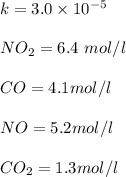

![=k.[NO_2]^2](/tpl/images/0723/2177/ff6c8.png) because the above given is the part of the second-order, which relates to
because the above given is the part of the second-order, which relates to 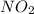 . In the zeros order the Carbon monoxide (CO) its reaction doesn't affect the rate.
. In the zeros order the Carbon monoxide (CO) its reaction doesn't affect the rate.