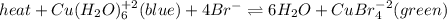The following equilibrium is formed when copper and bromide ions are placed in a solution:
heat + Cu(H2O)6 ^+2 (blue) + 4Br- <--> 6H2O + CuBr4^-2 (green)
A) answer the following questions when KBr is added to the solution:
1. What will happen to the equilibrium?
2. What will be the color of the solution?
3. Will the solution be hotter or cooler? Explain.
B) What will be the color of the solution when the solution is heated?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3


Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2
You know the right answer?
The following equilibrium is formed when copper and bromide ions are placed in a solution:
heat + C...
Questions

Biology, 28.07.2019 19:00



Social Studies, 28.07.2019 19:00



Mathematics, 28.07.2019 19:00

Mathematics, 28.07.2019 19:00




Physics, 28.07.2019 19:00

Physics, 28.07.2019 19:00

English, 28.07.2019 19:00

Mathematics, 28.07.2019 19:00

History, 28.07.2019 19:00

History, 28.07.2019 19:00



Mathematics, 28.07.2019 19:00