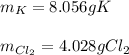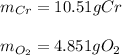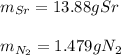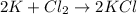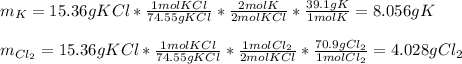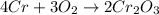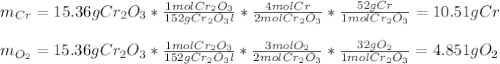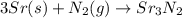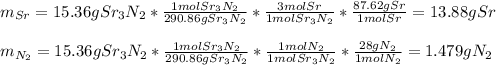Chemistry, 19.08.2020 05:01 momoney5746
For each of the following reactions calculate the mass (in grams) of both the reactants that are required to form 15.39g of the following products.
a. 2K(s) + Cl2(g) → 2Cl(aq)
b. 4Cr(s) + 302(g) → 2Cr2O3(s)
c. 35r(s) + N2(g) → SraNa(s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1


Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1
You know the right answer?
For each of the following reactions calculate the mass (in grams) of both the reactants that are req...
Questions

Business, 08.01.2022 03:50





Mathematics, 08.01.2022 03:50

Mathematics, 08.01.2022 03:50

Biology, 08.01.2022 03:50

Mathematics, 08.01.2022 03:50


Mathematics, 08.01.2022 03:50

Mathematics, 08.01.2022 03:50

Mathematics, 08.01.2022 03:50

Biology, 08.01.2022 03:50


History, 08.01.2022 03:50

Chemistry, 08.01.2022 03:50



Mathematics, 08.01.2022 04:00