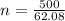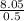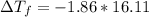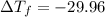Chemistry, 19.08.2020 05:01 Serenaark2834
Calculate the freezing point of a solution of 500.0 g of ethylene glycol dissovled in 500g water. Kf = 1.86 degrees C/m and Kb (which my instructor said was just extraneous info that is not used here) is 0.512 degrees C/m.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1


Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1
You know the right answer?
Calculate the freezing point of a solution of 500.0 g of ethylene glycol dissovled in 500g water. Kf...
Questions

Chemistry, 01.12.2020 05:40

Chemistry, 01.12.2020 05:40




Business, 01.12.2020 05:40

Mathematics, 01.12.2020 05:40


Geography, 01.12.2020 05:40

Mathematics, 01.12.2020 05:40

Computers and Technology, 01.12.2020 05:40

History, 01.12.2020 05:40

Mathematics, 01.12.2020 05:40





Mathematics, 01.12.2020 05:40

Mathematics, 01.12.2020 05:40

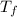 = -29.96 °C
= -29.96 °C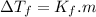
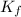 is the molal freezing-point depression constant
is the molal freezing-point depression constant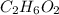 ) in the solution:
) in the solution: