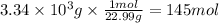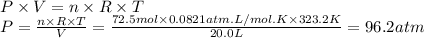Solid sodium reacts with liquid water to form hydrogen gas according to the equation 2 Na(s) + 2 H₂O(l) → 2 NaOH(aq) + H₂(g) What is the pressure of hydrogen gas in the 20.0 L headspace of a reactor vessel when 3.34 kg sodium is reacted with excess water at 50.0°C?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1
You know the right answer?
Solid sodium reacts with liquid water to form hydrogen gas according to the equation 2 Na(s) + 2 H₂O...
Questions


Chemistry, 02.04.2021 19:50



Mathematics, 02.04.2021 19:50

Mathematics, 02.04.2021 19:50


Mathematics, 02.04.2021 19:50


Social Studies, 02.04.2021 19:50

Arts, 02.04.2021 19:50

English, 02.04.2021 19:50

Computers and Technology, 02.04.2021 19:50

Mathematics, 02.04.2021 19:50




Biology, 02.04.2021 19:50

Mathematics, 02.04.2021 19:50

Mathematics, 02.04.2021 19:50