
Chemistry, 25.08.2020 01:01 HalpMahOnMahH0meW0rk
In an experiment, 0.2 mol of copper atoms was dissolved in nitric acid to produce copper(ii)nitrate.
a)Calculate the volume of gas measured at r. t.p, produced in this reaction
b)calculatr the maximum mass of copper(ii)nitrate, produced in this reaction
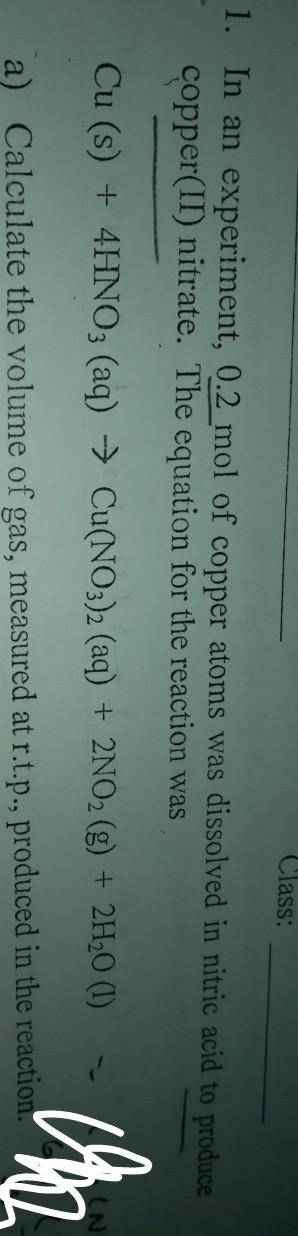

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

You know the right answer?
In an experiment, 0.2 mol of copper atoms was dissolved in nitric acid to produce copper(ii)nitrate....
Questions

Engineering, 23.05.2021 21:50



English, 23.05.2021 21:50



Mathematics, 23.05.2021 21:50

Business, 23.05.2021 21:50


Mathematics, 23.05.2021 21:50

Chemistry, 23.05.2021 21:50

Mathematics, 23.05.2021 21:50






Mathematics, 23.05.2021 21:50

Mathematics, 23.05.2021 21:50




