
Chemistry, 26.08.2020 05:01 reneebrown017
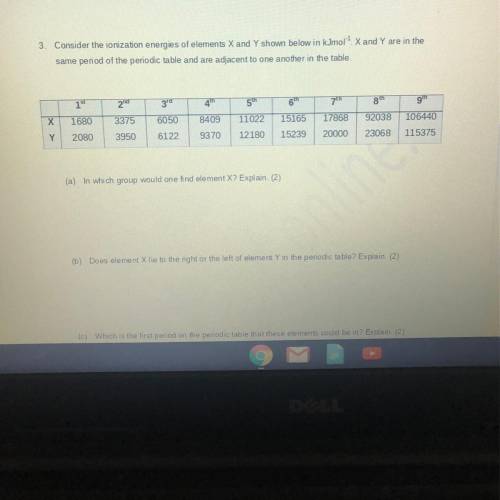
3. Consider the ionization energies of elements X and Y shown below in kJmol X and Y are in the
same period of the periodic table and are adjacent to one another in the table
2
30
4
5
6
7
8
9
1
x 1680
Y 2080
3375
6050
8409
17868
92038
106440
11022
12180
15165
15239
3950
6122
9370
20000
23068
115375
(a) In which group would one find element X? Explain. (2)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

You know the right answer?
3. Consider the ionization energies of elements X and Y shown below in kJmol X and Y are in the
sam...
Questions


Biology, 01.12.2019 05:31


Mathematics, 01.12.2019 05:31


Mathematics, 01.12.2019 05:31


Mathematics, 01.12.2019 05:31

Mathematics, 01.12.2019 05:31



Mathematics, 01.12.2019 05:31




Mathematics, 01.12.2019 05:31


Health, 01.12.2019 05:31




