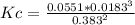A sample of ammonia gas was allowed to come to equilibrium at 400 K. 2NH3(g) <---> N2(g) 3H2(g) At equilibrium, it was found that the concentration of H2 was 0.0551 M, the concentration of N2 was 0.0183 M, and the concentration of NH3 was 0.383 M. What is Kc for this equilibrium

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3


Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
A sample of ammonia gas was allowed to come to equilibrium at 400 K. 2NH3(g) <---> N2(g) 3H2(g...
Questions


History, 28.02.2020 03:59

Mathematics, 28.02.2020 03:59

Mathematics, 28.02.2020 03:59


Mathematics, 28.02.2020 03:59

Mathematics, 28.02.2020 03:59


Mathematics, 28.02.2020 03:59

Mathematics, 28.02.2020 03:59




Mathematics, 28.02.2020 04:00




Mathematics, 28.02.2020 04:00


![Kc=\frac{[C]^{c}*[D]^{d} }{[A]^{a} *[B]^{b} }](/tpl/images/0729/9313/c290c.png)
![Kc=\frac{[N_{2} ]*[H_{2} ]^{3} }{[NH_{3} ]^{2} }](/tpl/images/0729/9313/3ff30.png)