
Chemistry, 27.08.2020 02:01 lexiremmickp6mlxe
The pH of a solution is 8.83±0.048.83±0.04 . What is the concentration of H+H+ in the solution and its absolute uncertainty?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2
You know the right answer?
The pH of a solution is 8.83±0.048.83±0.04 . What is the concentration of H+H+ in the solution and i...
Questions

Mathematics, 03.03.2021 18:50





Mathematics, 03.03.2021 19:00

Biology, 03.03.2021 19:00


Mathematics, 03.03.2021 19:00

Mathematics, 03.03.2021 19:00

Mathematics, 03.03.2021 19:00



Mathematics, 03.03.2021 19:00

Social Studies, 03.03.2021 19:00


Mathematics, 03.03.2021 19:00


History, 03.03.2021 19:00

Computers and Technology, 03.03.2021 19:00

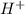 is 1.48 ×
is 1.48 × 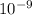 M
M![[{H^{+}]](/tpl/images/0730/8214/7a0a5.png) is ±0.12 ×
is ±0.12 × ![-log_{10}[{H^{+}]](/tpl/images/0730/8214/16021.png)
![[H^{+}] = 10^{-pH}](/tpl/images/0730/8214/241df.png)
![[{H^{+}] = 10^{-8.83}](/tpl/images/0730/8214/2a5c0.png)
![[{H^{+}] = 1.4791](/tpl/images/0730/8214/fde5a.png) ×
× ![[{H^{+}] = 1.48](/tpl/images/0730/8214/3c5a4.png) ×
× ![U_{[H^{+}] }](/tpl/images/0730/8214/3bd1c.png) ) from the equation
) from the equation ![U_{[H^{+}] } = 2.303 \\](/tpl/images/0730/8214/86a61.png) ×
× ![{[H^{+}] }](/tpl/images/0730/8214/75c28.png) ×
× 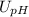
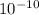 M
M

