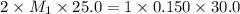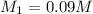Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
A 25.0 mL sample of sulfuric acid is titrated with 30.0 mL of 0.150 M sodium hydroxide. Calculate th...
Questions



Mathematics, 20.09.2021 15:40



Geography, 20.09.2021 15:40



Mathematics, 20.09.2021 15:40


Mathematics, 20.09.2021 15:50


Mathematics, 20.09.2021 15:50

Mathematics, 20.09.2021 15:50

Mathematics, 20.09.2021 15:50

Mathematics, 20.09.2021 15:50


English, 20.09.2021 15:50


Mathematics, 20.09.2021 15:50

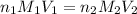
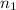 = basicity of sulphuric acid
= basicity of sulphuric acid 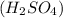 = 2
= 2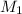 = Molarity of sulphuric acid
= Molarity of sulphuric acid 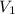 = volume of sulphuric acid
= volume of sulphuric acid 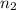 = acidity of sodium hydroxide
= acidity of sodium hydroxide 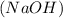 = 1
= 1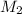 = Molarity of sodium hydroxide
= Molarity of sodium hydroxide 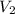 = volume of sodium hydroxide
= volume of sodium hydroxide