
Chemistry, 26.08.2020 18:01 blueval3tine
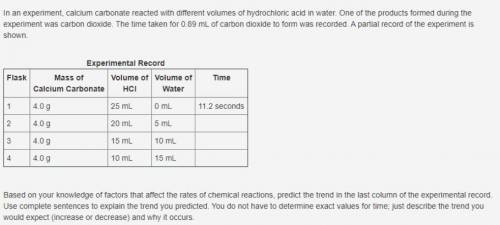
In an experiment, calcium carbonate reacted with different volumes of hydrochloric acid in water. One of the products formed during the experiment was carbon dioxide. The time taken for 0.89 mL of carbon dioxide to form was recorded. A partial record of the experiment is shown. Experimental Record Flask Mass of Calcium Carbonate Volume of HCl Volume of Water Time 1 4.0 g 25 mL 0 mL 11.2 seconds 2 4.0 g 20 mL 5 mL 3 4.0 g 15 mL 10 mL 4 4.0 g 10 mL 15 mL

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

You know the right answer?
In an experiment, calcium carbonate reacted with different volumes of hydrochloric acid in water. On...
Questions



English, 24.03.2021 01:20


History, 24.03.2021 01:20




Mathematics, 24.03.2021 01:20



Mathematics, 24.03.2021 01:20

Mathematics, 24.03.2021 01:20

Social Studies, 24.03.2021 01:20

Mathematics, 24.03.2021 01:30

Geography, 24.03.2021 01:30

History, 24.03.2021 01:30

Chemistry, 24.03.2021 01:30





