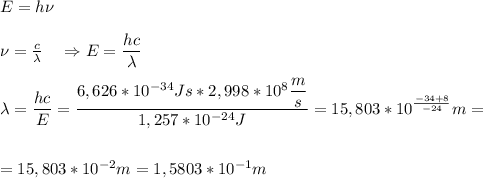Chemistry, 22.10.2019 02:00 FavvBella84
Calculate the wavelength of a photon having energy of 1.257 x 10-24 joules. (planck’s constant is 6.626 x 10-34 joule seconds; the speed of light is 2.998 x 108 m/s)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1


Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
Calculate the wavelength of a photon having energy of 1.257 x 10-24 joules. (planck’s constant is 6....
Questions

Engineering, 20.02.2021 14:40

Mathematics, 20.02.2021 14:40


English, 20.02.2021 14:40

Mathematics, 20.02.2021 14:40

Business, 20.02.2021 14:40

English, 20.02.2021 14:40

Arts, 20.02.2021 14:40





Computers and Technology, 20.02.2021 14:50

Physics, 20.02.2021 14:50

Mathematics, 20.02.2021 14:50



Medicine, 20.02.2021 14:50


Computers and Technology, 20.02.2021 14:50