
Chemistry, 27.08.2020 21:01 oksanabkrot
A reaction between 7.0 g of copper(II) oxide and 50 mL of 0.20 M nitric acid produces
copper(II) nitrate, Cu(NO3)2 and water.
(c) Determine the limiting reactant.
(d) Calculate the mass of excess reactant after the reaction.
(ANS: 6.6068g)
(e) Determine the percentage yield if the actual mass of copper (II) nitrate obtained from
the reaction is 0.85 g.
(ANS: 90.64%)
How to get the mass of HNO3 from here? I only managed to get mass of NO3 based on the molarity formula. thanks!

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:02
If the empirical formula of a compound is known what is needed in order to determine the molecular formula a) the coordination numbers b)the molecular geometry c) the molar mass
Answers: 1

Chemistry, 21.06.2019 12:30
Which of the following can be used to measure electricity
Answers: 1

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1
You know the right answer?
A reaction between 7.0 g of copper(II) oxide and 50 mL of 0.20 M nitric acid produces
copper(II) ni...
Questions



Biology, 08.11.2019 18:31


History, 08.11.2019 18:31

Mathematics, 08.11.2019 18:31

History, 08.11.2019 18:31



Biology, 08.11.2019 18:31

Mathematics, 08.11.2019 18:31

Mathematics, 08.11.2019 18:31

Health, 08.11.2019 18:31



Biology, 08.11.2019 18:31


Mathematics, 08.11.2019 18:31

Mathematics, 08.11.2019 18:31

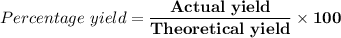
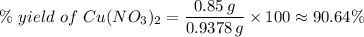
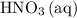 is the limiting reactant.
is the limiting reactant.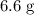 of
of 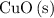 will be in excess.
will be in excess.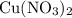 is approximately
is approximately 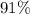 . (Rounded to two significant figures, as in other quantities in the question.)
. (Rounded to two significant figures, as in other quantities in the question.)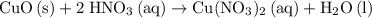 .
.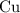 :
: 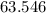 .
. :
: 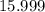 .
.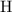 :
: 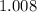 .
.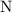 :
: 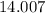 .
.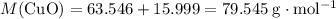 .
.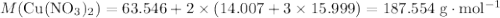 .Limiting Reactant
.Limiting Reactant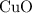 and
and 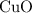 is the limiting one. In other words, assume that all the
is the limiting one. In other words, assume that all the 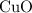 is consumed before
is consumed before 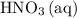 was.
was. 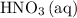 would be required to convert all that
would be required to convert all that 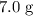 of
of 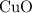 to
to 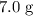 of
of 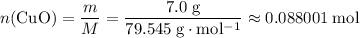 .
.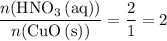 .
.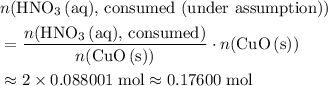 .
. 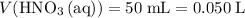 .
.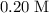 solution:
solution: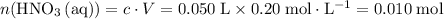 .
.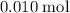 of
of 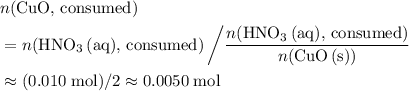 .
.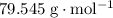 . Therefore, the mass of that
. Therefore, the mass of that 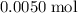 formula units of
formula units of 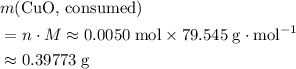 .
.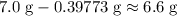 of
of 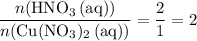 .
.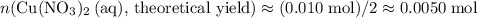 .
.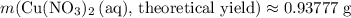 .
.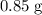 :
: .
.

