
Chemistry, 28.08.2020 20:01 eshaesmot12345
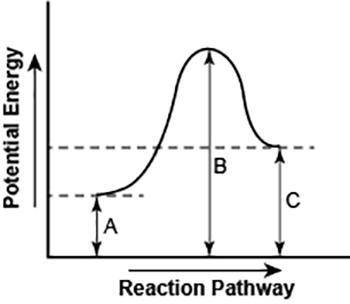
Part 1: Describe how you can determine the total change in enthalpy and activation energy from the diagram, and if each is positive or negative. Part 2: Describe how the curve will look if the reaction was exothermic. Be sure to mention changes in the potential energies of the reactants and products and the sign changes of the enthalpy.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1
You know the right answer?
Part 1: Describe how you can determine the total change in enthalpy and activation energy from the d...
Questions


Social Studies, 08.06.2021 14:00


Social Studies, 08.06.2021 14:00

Mathematics, 08.06.2021 14:00

Chemistry, 08.06.2021 14:00

English, 08.06.2021 14:00




History, 08.06.2021 14:00



Computers and Technology, 08.06.2021 14:00




World Languages, 08.06.2021 14:00

Mathematics, 08.06.2021 14:00

Chemistry, 08.06.2021 14:00



