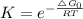Chemistry, 28.08.2020 16:01 j1theking18
Choose the best answer below. Which of the following reactions will have the largest equilibrium constant at 298 K?
a) 302(g) → 203(9) AGOrxn = +326 kJ
b) Mg(s) + N20(g) → Mgo(s) + N2(g) AG9rxn = -673.0 kJ
c) 2Hg(g) + O2(g) → 2HgO(s) AGºrx = -180.8 kJ
d) CaCO3(s) » Cao(s) + CO2(g) AG = +131.1 kJ
It is not possible to determine the reaction with the largest equilibrium constant using the given information.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
Choose the best answer below. Which of the following reactions will have the largest equilibrium con...
Questions

Mathematics, 21.05.2021 16:20





Mathematics, 21.05.2021 16:20



Mathematics, 21.05.2021 16:20


Chemistry, 21.05.2021 16:20

Computers and Technology, 21.05.2021 16:20

Mathematics, 21.05.2021 16:20

Mathematics, 21.05.2021 16:20


Social Studies, 21.05.2021 16:20


Chemistry, 21.05.2021 16:20


Mathematics, 21.05.2021 16:20