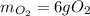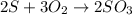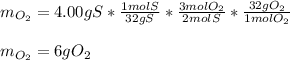Chemistry, 31.08.2020 07:01 brialevy2283
When 5.00 g of sulfur are combined with 5.00 g of oxygen, 10.00 g of sulfur dioxide (SO2) are formed. What mass of oxygen would be required to convert 4.00 g of sulfur into sulfur trioxide (SO3)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1


Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
When 5.00 g of sulfur are combined with 5.00 g of oxygen, 10.00 g of sulfur dioxide (SO2) are formed...
Questions

Mathematics, 05.05.2021 16:50






Computers and Technology, 05.05.2021 16:50

Chemistry, 05.05.2021 16:50




Mathematics, 05.05.2021 16:50

History, 05.05.2021 16:50




Mathematics, 05.05.2021 16:50

Mathematics, 05.05.2021 16:50

Mathematics, 05.05.2021 16:50

Business, 05.05.2021 16:50