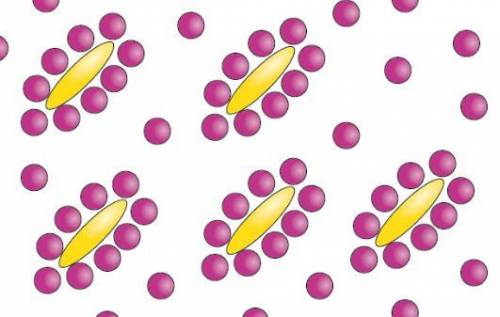
Behold a mixture of oily/hydrophobic (yellow) and water (purplish) molecules. recall that hydrophobic regions are ones with few or no partial charges--so there's nothing for water's hydrogen interaction donors or acceptors to 'play with'. so! how many of the water molecules are 'stuck' contacting at least part of a hydrophobic molecule in this arrangement?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1
You know the right answer?
Behold a mixture of oily/hydrophobic (yellow) and water (purplish) molecules. recall that hydrophobi...
Questions



Mathematics, 15.10.2019 09:30

Biology, 15.10.2019 09:30



Biology, 15.10.2019 09:30




Mathematics, 15.10.2019 09:30

Mathematics, 15.10.2019 09:30



Mathematics, 15.10.2019 09:30




Physics, 15.10.2019 09:30

Physics, 15.10.2019 09:30