Chemistry, 02.09.2020 23:01 justhereforanswers13
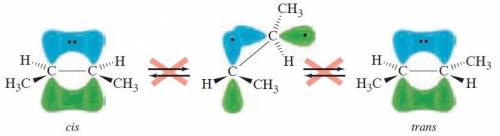
Rotation about a carbon-carbon double bond does not readily occur because: .1) the overlap of the p orbitals of the carbon-carbon π bond would be lost2) the double bond is much shorter and therefore more difficult to rotate3) the overlap of the sp2 orbitals of the carbon-carbon σ bond would be lost4) the double bond is much stronger and therefore more difficult to rotate

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1

Chemistry, 23.06.2019 09:00
Which of the following are in a chemical family a. ca, sc, k b. cu, ag, au c. so, ge, sb
Answers: 1

Chemistry, 23.06.2019 09:30
The allotropes of carbon include a variety of structures that include three-dimensional tetrahedral lattices, planes of hexagonal rings, cylindrical tubes of hexagonal rings, and spheres of five- and six-membered rings. similar shapes of network covalent atomic solids are possible with carbon nitride, boron, and pure silicon (e.g., silicene is a graphene-like allotrope of pure silicon). in contrast, silicates exist as either highly ordered or amorphous (more random) three-dimensional lattices. what could explain why there are there no naturally occurring sheets, stacked sheets, cylindrical tubes, or spheres of network covalent atomic solids composed of silicon and oxygen (sio2)? would pure silicate structures make good lubricants or good electrical conductors?
Answers: 3
You know the right answer?
Rotation about a carbon-carbon double bond does not readily occur because: .1) the overlap of the p...
Questions

English, 20.01.2021 19:20

Physics, 20.01.2021 19:20



History, 20.01.2021 19:20

Mathematics, 20.01.2021 19:20

English, 20.01.2021 19:20

English, 20.01.2021 19:20

Social Studies, 20.01.2021 19:20

Mathematics, 20.01.2021 19:20

Mathematics, 20.01.2021 19:20




Biology, 20.01.2021 19:20


English, 20.01.2021 19:20


Arts, 20.01.2021 19:20