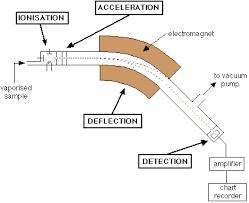
Chemistry, 02.09.2020 01:01 bbyniah123
The mass spectrum of a sample of a pure element is shown above. Based on the data, the peak at 26amu represents an isotope of which of the following elements?
A) Al with 13 neutrons
B) Mg with 14 neutrons
C) Fe with 26 neutrons
D) Ti with 26 neutrons


Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1
You know the right answer?
The mass spectrum of a sample of a pure element is shown above. Based on the data, the peak at 26amu...
Questions



Mathematics, 24.02.2020 23:04

Computers and Technology, 24.02.2020 23:05

English, 24.02.2020 23:05








Mathematics, 24.02.2020 23:05



Mathematics, 24.02.2020 23:06


Mathematics, 24.02.2020 23:06