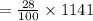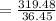Chemistry, 03.09.2020 01:01 baleycardell9901
The mass percentage of hydrochloric acid within a solution is 28.00%28.00% . Given that the density of this solution is 1.1411.141 g/mL, find the molarity of the solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3
You know the right answer?
The mass percentage of hydrochloric acid within a solution is 28.00%28.00% . Given that the density...
Questions


Social Studies, 19.09.2019 19:00

Mathematics, 19.09.2019 19:00



English, 19.09.2019 19:00


Social Studies, 19.09.2019 19:00


Mathematics, 19.09.2019 19:00


Chemistry, 19.09.2019 19:00

Mathematics, 19.09.2019 19:00



History, 19.09.2019 19:00

Biology, 19.09.2019 19:00

Biology, 19.09.2019 19:00

History, 19.09.2019 19:00