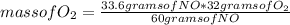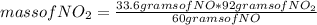Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1


Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
If 33.6 g of NO and 26.9 g of O₂ react together, what is the mass in grams of NO₂ that can be formed...
Questions



Business, 27.07.2019 17:10





Mathematics, 27.07.2019 17:10


Mathematics, 27.07.2019 17:10

Social Studies, 27.07.2019 17:10


Social Studies, 27.07.2019 17:10


History, 27.07.2019 17:10