

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
The weak acid HA is 4 % ionized (dissociated) in a 0.30 M solution. A. What is Ka for this acid?B. W...
Questions






Social Studies, 04.05.2020 22:53

World Languages, 04.05.2020 22:53







Chemistry, 04.05.2020 22:53

Chemistry, 04.05.2020 22:53





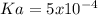
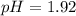
![\% ionization=\frac{[H^+]}{[HA]}*100\%](/tpl/images/0739/3957/49c2b.png)
![[H^+]=\frac{\% ionization*[HA]}{100\%} =\frac{4\%*0.30M}{100\%}=0.012M](/tpl/images/0739/3957/75e88.png)
![Ka=\frac{[H^+][A^-]}{[HA]} =\frac{0.012M*0.012M}{0.30M-0.012M}\\ \\Ka=5x10^{-4}](/tpl/images/0739/3957/ccddd.png)
![pH=-log([H^+])=-log(0.012)\\\\pH=1.92](/tpl/images/0739/3957/80a40.png)


