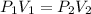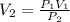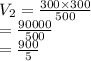Chemistry, 03.09.2020 14:01 strodersage
7. A gas has a volume of 300 mL at 300 mm Hg. What will its volume be if the pressure is changed to 500 mm Hg?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
You know the right answer?
7. A gas has a volume of 300 mL at 300 mm Hg. What will its volume be if the pressure is changed to...
Questions

Mathematics, 22.08.2019 04:50

Mathematics, 22.08.2019 04:50

Biology, 22.08.2019 04:50


Mathematics, 22.08.2019 04:50

History, 22.08.2019 04:50


English, 22.08.2019 04:50


Mathematics, 22.08.2019 04:50




Social Studies, 22.08.2019 04:50

History, 22.08.2019 04:50


Mathematics, 22.08.2019 04:50