
Chemistry, 03.09.2020 21:01 ilovecatsomuchlolol
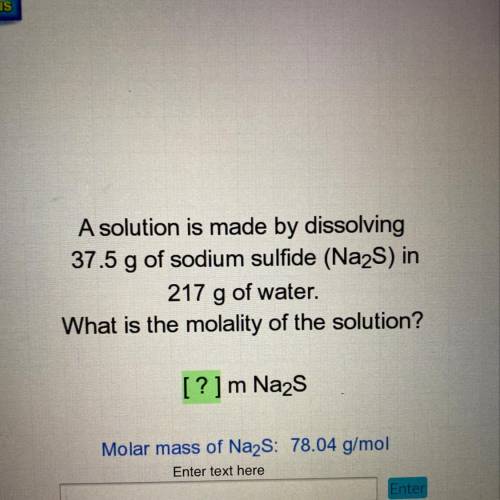
A solution is made by dissolving
37.5 g of sodium sulfide (Na2S) in
217 g of water.
What is the molality of the solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2
You know the right answer?
A solution is made by dissolving
37.5 g of sodium sulfide (Na2S) in
217 g of water.
Wha...
217 g of water.
Wha...
Questions

Mathematics, 17.09.2019 20:00

English, 17.09.2019 20:00

Mathematics, 17.09.2019 20:00


History, 17.09.2019 20:00


Mathematics, 17.09.2019 20:00



Mathematics, 17.09.2019 20:00




Mathematics, 17.09.2019 20:00

Social Studies, 17.09.2019 20:00


History, 17.09.2019 20:00


English, 17.09.2019 20:00



