
Chemistry, 03.09.2020 07:01 qawsedrftgyh3487
In an ion with an unknown charge, the total mass of all the electrons was determined to be 1.640 x 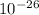 g, the total mass of its protons was 4.015 x
g, the total mass of its protons was 4.015 x 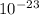 g, and the total mass of its neutrons was 4.690 x
g, and the total mass of its neutrons was 4.690 x 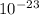 g. What is the identity, charge, and mass number of this ion? Electron one mass = 9.109 x
g. What is the identity, charge, and mass number of this ion? Electron one mass = 9.109 x 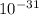 Proton one mass = 1.673 x
Proton one mass = 1.673 x 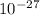 Neutron one mass = 1.675 x
Neutron one mass = 1.675 x 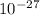

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
You know the right answer?
In an ion with an unknown charge, the total mass of all the electrons was determined to be 1.640 x...
Questions


History, 20.10.2020 02:01




Mathematics, 20.10.2020 02:01


Mathematics, 20.10.2020 02:01



Mathematics, 20.10.2020 02:01




Advanced Placement (AP), 20.10.2020 02:01

Physics, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01


Chemistry, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01



