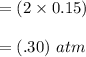Chemistry, 04.09.2020 21:01 hjamileth77
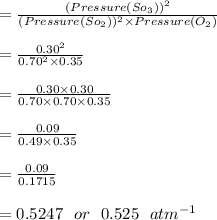
Some SO2 and O2 are mixed together in a flask at 1100 K in such a way ,that at the instant of mixing, their partial pressures are, respectively, 1.00 atm and 0.500 atm. When the system comes to equilibrium at 1100 K, the total pressure in the flask is found to be 1.35 atm. Given: 2SO2(g) + O2(g) ⇌ 2SO3(g); ΔrH = − 198.2 kJ. mol-1 1.1 Calculate Kp at 1100 K

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2
You know the right answer?
Some SO2 and O2 are mixed together in a flask at 1100 K in such a way ,that at the instant of mixing...
Questions

English, 03.01.2020 07:31








History, 03.01.2020 07:31




Mathematics, 03.01.2020 07:31




Mathematics, 03.01.2020 07:31


Mathematics, 03.01.2020 07:31

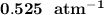 "
"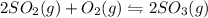
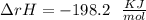
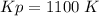
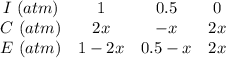
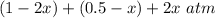
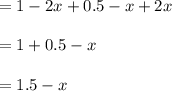
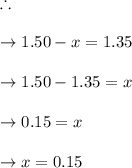
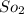 :
: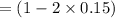
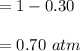
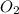 :
: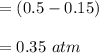
 :
: