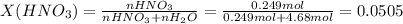Chemistry, 04.09.2020 23:01 0465834wu1
Calculate the mole fraction of nitric acid of a(n) 15.7% (by mass) aqueous solution of nitric acid. Calculate the mole fraction of nitric acid of a(n) 15.7% (by mass) aqueous solution of nitric acid. 2.56×10−2 0.102 5.33×10−2 5.11×10−2 The density of the solution is needed to solve the problem.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
You know the right answer?
Calculate the mole fraction of nitric acid of a(n) 15.7% (by mass) aqueous solution of nitric acid....
Questions


Mathematics, 17.02.2021 02:30

English, 17.02.2021 02:30


Physics, 17.02.2021 02:30


History, 17.02.2021 02:30

Mathematics, 17.02.2021 02:30



Mathematics, 17.02.2021 02:30

English, 17.02.2021 02:30


Spanish, 17.02.2021 02:30

History, 17.02.2021 02:30


Mathematics, 17.02.2021 02:30

Mathematics, 17.02.2021 02:30