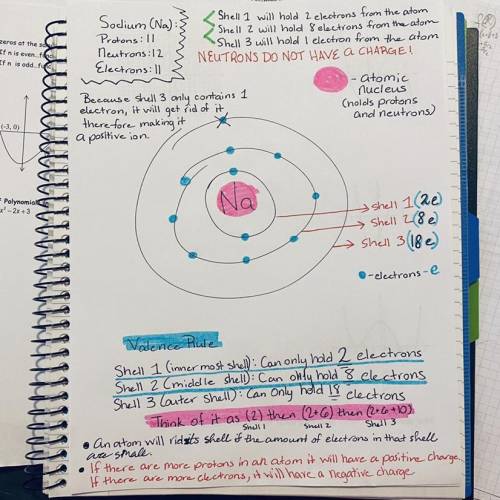
Let’s take a look at sodium (Na) and chlorine (Cl). Draw what I am describing, and you will see it better. A sodium atom has how many protons? A sodium atom has how many electrons? How many electrons will go in the first shell? How many in the second shell? How many in the third? Now draw this out on the diagram in Figure 2.1, and take a look at it, in particular the third (valence) shell. We know that Na requires eight electrons in its valence shell to become stable. But how many does it have? So, to fill this shell, will it be easier for sodium to steal seven more electrons from another atom, or will it be easier for sodium to give up that one electron and get rid of that third shell? Sodium is simply going to give away that last electron. This means that it will lose an electron (negative charge) but will keep the same number of protons (positive charges). What will the sodium ion’s overall charge be now? _

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3


Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Let’s take a look at sodium (Na) and chlorine (Cl). Draw what I am describing, and you will see it b...
Questions


French, 23.09.2019 14:00


Social Studies, 23.09.2019 14:00

Mathematics, 23.09.2019 14:00

Mathematics, 23.09.2019 14:00

Mathematics, 23.09.2019 14:00

Mathematics, 23.09.2019 14:00


Health, 23.09.2019 14:00


English, 23.09.2019 14:00


Mathematics, 23.09.2019 14:00

Biology, 23.09.2019 14:00

Physics, 23.09.2019 14:00

Chemistry, 23.09.2019 14:00


Mathematics, 23.09.2019 14:00