Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
At a certain temperature, 0.338 mol CH and 0.831 mol HO is placed in a 3.50 L container.
CH(g)+2HO(...
Questions

Mathematics, 08.01.2020 18:31


Mathematics, 08.01.2020 18:31



Mathematics, 08.01.2020 18:31

Biology, 08.01.2020 18:31

Mathematics, 08.01.2020 18:31

Physics, 08.01.2020 18:31


History, 08.01.2020 18:31





Mathematics, 08.01.2020 18:31


Mathematics, 08.01.2020 18:31

Mathematics, 08.01.2020 18:31

Mathematics, 08.01.2020 18:31

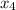 and 0.831 mol H
and 0.831 mol H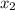 O is placed in a 3.50 L container.
CH
O is placed in a 3.50 L container.
CH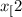 (g) At equilibrium, 6.51 g CO
(g) At equilibrium, 6.51 g CO