
Chemistry, 05.09.2020 07:01 antboozeoszxej
Assume that the chemical reaction shown started out having a total of 15g of potassium and water How much potassium hydroxide and hydrogen gas will be produced by the chemical reaction? Show your work


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3
You know the right answer?
Assume that the chemical reaction shown started out having a total of 15g of potassium and water How...
Questions

Mathematics, 11.05.2021 06:20

Mathematics, 11.05.2021 06:20


Mathematics, 11.05.2021 06:20

Spanish, 11.05.2021 06:20

Mathematics, 11.05.2021 06:20

Mathematics, 11.05.2021 06:20





Mathematics, 11.05.2021 06:20

Mathematics, 11.05.2021 06:20

Mathematics, 11.05.2021 06:20


Arts, 11.05.2021 06:20

Physics, 11.05.2021 06:20

Biology, 11.05.2021 06:20





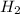 .
.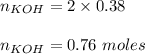

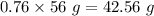 .
. .
.

