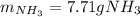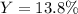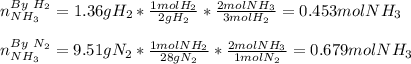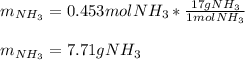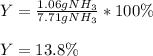Chemistry, 05.09.2020 22:01 Lindsay882
3H2(g)+N2(g)→2NH3(g) 1.36 g H2 is allowed to react with 9.51 g N2, producing 1.06 g NH3 1.) What is the theoretical yield in grams for this reaction under the given conditions? 2.)What is the percent yield for this reaction under the given conditions?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

You know the right answer?
3H2(g)+N2(g)→2NH3(g) 1.36 g H2 is allowed to react with 9.51 g N2, producing 1.06 g NH3 1.) What is...
Questions

Arts, 04.02.2021 22:00

Mathematics, 04.02.2021 22:00


History, 04.02.2021 22:00

Mathematics, 04.02.2021 22:00

Chemistry, 04.02.2021 22:00

Arts, 04.02.2021 22:00


Mathematics, 04.02.2021 22:00



Mathematics, 04.02.2021 22:00

Mathematics, 04.02.2021 22:00

Mathematics, 04.02.2021 22:00






Mathematics, 04.02.2021 22:00