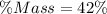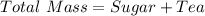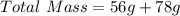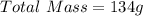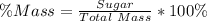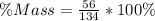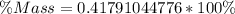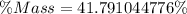Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
You are making sweet tea. You add 56 g of sugar into 78 g of tea. What is the
mass percent of sugar...
Questions


Mathematics, 03.03.2021 01:00


Mathematics, 03.03.2021 01:00

Arts, 03.03.2021 01:00

History, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00




Mathematics, 03.03.2021 01:00


History, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00


Advanced Placement (AP), 03.03.2021 01:00

Social Studies, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00