
A piece of a metal having a mass of 150.0000 grams is heated to 99.3 9C. When the hot metal is submerged in 50.000 grams of water originally at 21.3 oC, the final temperature of the water and metal is 48.4 pC. Determine the heat flow (q) of the water in Joules Record your answer to the correct number of significant figures
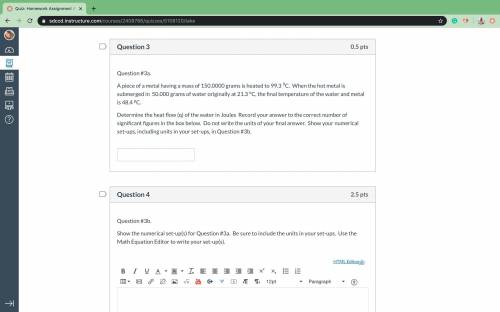

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2
You know the right answer?
A piece of a metal having a mass of 150.0000 grams is heated to 99.3 9C. When the hot metal is subme...
Questions


Biology, 10.03.2020 00:08



Health, 10.03.2020 00:08

Mathematics, 10.03.2020 00:08


History, 10.03.2020 00:08



Computers and Technology, 10.03.2020 00:08



History, 10.03.2020 00:08


Mathematics, 10.03.2020 00:08



Law, 10.03.2020 00:08



