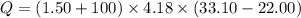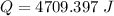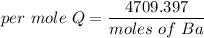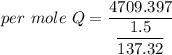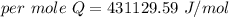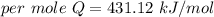Chemistry, 07.09.2020 18:01 breanna7667
When 1.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere, the reaction shown below occurs and the temperature of the resulting solution rises from 22.00°C to 33.10°C. If the specific heat of the solution is 4.18 J/(g ∙ °C), calculate for the reaction, as written. Ba(s) + 2 H2O(l) → Ba(OH)2(aq) + H2(g)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
When 1.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere, the reacti...
Questions

Medicine, 12.11.2020 05:40



Mathematics, 12.11.2020 05:40

History, 12.11.2020 05:40

Social Studies, 12.11.2020 05:40

Spanish, 12.11.2020 05:40


English, 12.11.2020 05:40

English, 12.11.2020 05:40


English, 12.11.2020 05:40

Biology, 12.11.2020 05:40

Mathematics, 12.11.2020 05:40

Mathematics, 12.11.2020 05:40

Mathematics, 12.11.2020 05:40

History, 12.11.2020 05:40



Business, 12.11.2020 05:40

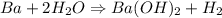
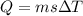
 temperature
temperature