
Chemistry, 07.09.2020 21:01 itzdryoshi
The radius of a copper atom is 1.3⋅10^−10m. How many times N can you divide evenly a 11.1 cm long line of copper atoms until it is reduced to a single copper atom?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
The radius of a copper atom is 1.3⋅10^−10m. How many times N can you divide evenly a 11.1 cm long li...
Questions


Mathematics, 16.11.2020 22:10

Mathematics, 16.11.2020 22:10

Mathematics, 16.11.2020 22:10


Mathematics, 16.11.2020 22:10





Arts, 16.11.2020 22:10



SAT, 16.11.2020 22:10

Chemistry, 16.11.2020 22:10

Computers and Technology, 16.11.2020 22:10


Mathematics, 16.11.2020 22:10


Mathematics, 16.11.2020 22:10

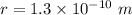 .
.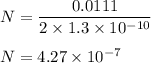
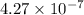 .
.

