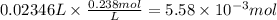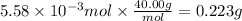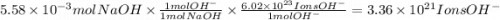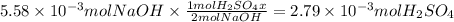Chemistry, 08.09.2020 14:01 amandaestevez030
Consider a 0.238 M aqueous solution of sodium hydroxide, NaOH.
a. How many grams of NaOH are dissolved in 23.46 mL?
b. How many individual hydroxide ions (OH) are found in 23.46 mL?
c. How many moles of sulfuric acid, H2SO4, are neutralized by 23.46 mL of 0.238 M NaOH(aq)?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1



Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
Consider a 0.238 M aqueous solution of sodium hydroxide, NaOH.
a. How many grams of NaOH are dissol...
Questions

Mathematics, 23.03.2021 23:50

Mathematics, 23.03.2021 23:50

Mathematics, 23.03.2021 23:50



English, 23.03.2021 23:50

Mathematics, 23.03.2021 23:50

Mathematics, 23.03.2021 23:50




Chemistry, 23.03.2021 23:50

History, 23.03.2021 23:50



English, 23.03.2021 23:50


Mathematics, 23.03.2021 23:50