

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
The ground-state electronic configuration of the manganese atom, Mn, is:.
A) 1s2 2s2 2p6 3s2 3p6 4s...
Questions


Social Studies, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01

Social Studies, 02.10.2020 15:01


History, 02.10.2020 15:01





Mathematics, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01

Health, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01


Social Studies, 02.10.2020 15:01


Biology, 02.10.2020 15:01

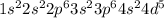 .
.

