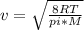Chemistry, 08.09.2020 15:01 trashbinkid
Four identical 1.0-L flasks contain the gases He, Cl2, CH4, and NH3, each at 0°C and 1 atm pressure. For which gas do the molecules have the highest average velocity?
a) He
b) Cl2
c) all gases the same
d) NH3

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3


Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
Four identical 1.0-L flasks contain the gases He, Cl2, CH4, and NH3, each at 0°C and 1 atm pressure....
Questions

Computers and Technology, 06.11.2020 18:40



Mathematics, 06.11.2020 18:40

Mathematics, 06.11.2020 18:40

Mathematics, 06.11.2020 18:40


Biology, 06.11.2020 18:40


History, 06.11.2020 18:40



Mathematics, 06.11.2020 18:40


Geography, 06.11.2020 18:50

Mathematics, 06.11.2020 18:50

History, 06.11.2020 18:50



Mathematics, 06.11.2020 18:50