
Chemistry, 09.09.2020 08:01 lwilliams28
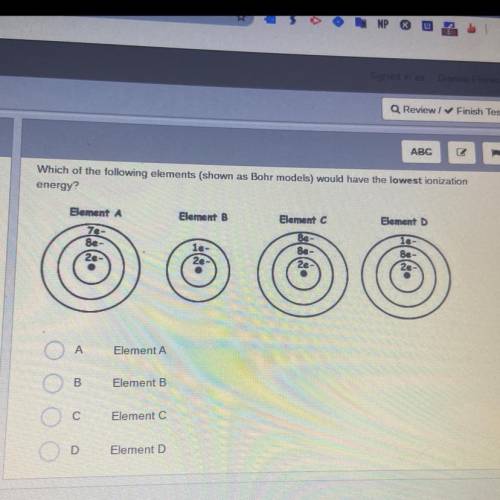
Which of the following elements (shown as Bohr models) would have the lowest ionization
energy?
Element A
Element B
Element a
Element D
ro
save
le-
7e-
8e-
2e
le-
Be
8e
2e
8e-
2e
Circuits
esign
.ce
riods of
device.
table
A
Element A
B
Element B
С
Element C
D
Element D

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2


Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
Which of the following elements (shown as Bohr models) would have the lowest ionization
energy?
Questions

Mathematics, 10.02.2021 22:20


Advanced Placement (AP), 10.02.2021 22:20



Mathematics, 10.02.2021 22:20


Mathematics, 10.02.2021 22:20


Social Studies, 10.02.2021 22:20


Mathematics, 10.02.2021 22:20

Mathematics, 10.02.2021 22:20



History, 10.02.2021 22:20

Chemistry, 10.02.2021 22:20



Mathematics, 10.02.2021 22:20



