Chemistry, 09.09.2020 01:01 oscardiazbet8803
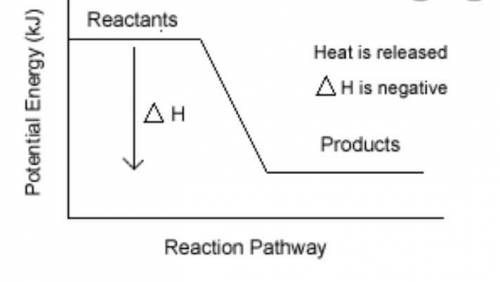
Burning of fuel in air is called combustion. CH4(g) + 2O2(g) CO2 (g)+ 2H2O(l) a) Calculate the heat of reaction for the above reaction by using the following bond energies . (3 Marks) C-H single bond is 412 kJ , O=O double bond is 496 kJ, C=O double bond is 743 kJ, H-O single bond is 463 kJ b) Draw the energy profile diagram for the above reaction .On your diagram label the • Products, Reactants • enthalpy for the reaction • activation energy, Ea.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1

Chemistry, 23.06.2019 10:10
Which orbitals form a pi bond? a. the s orbital and three p orbitals b. the s orbital and two p orbitals c. overlapping p orbitals d. overlapping hybrid orbitals
Answers: 2
You know the right answer?
Burning of fuel in air is called combustion. CH4(g) + 2O2(g) CO2 (g)+ 2H2O(l) a) Calculate the hea...
Questions

Mathematics, 03.05.2021 17:10



Biology, 03.05.2021 17:10

Mathematics, 03.05.2021 17:10




Mathematics, 03.05.2021 17:10

Mathematics, 03.05.2021 17:10


Mathematics, 03.05.2021 17:10

Mathematics, 03.05.2021 17:10


Physics, 03.05.2021 17:10


Computers and Technology, 03.05.2021 17:10

Mathematics, 03.05.2021 17:10