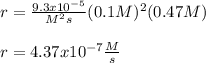The reaction 2NO(g) + O2(g) 2NO2(g) is second order in NO and first order in O2. When [NO] = 0.8 M and [O2] = 3.7 M, the observed rate of the reaction is 0.00022022 M/s. (a) What is the value of the rate constant? (d) What is the rate of reaction when [NO] = 0.1 M and [O2] = 0.47 M?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1

Chemistry, 23.06.2019 09:30
Which of the following is not a characteristic of a hydrogen bond? 1. it is responsible for the unusual physical properties of water. 2. it is weaker than a covalent bond. 3. it is stronger than other dipole-dipole interactions. 4. it can occur when hydrogen is covalently bound to very electronegative elements liks f, cl, br and i.
Answers: 1
You know the right answer?
The reaction 2NO(g) + O2(g) 2NO2(g) is second order in NO and first order in O2. When [NO] = 0.8 M a...
Questions



Social Studies, 09.04.2020 18:34

Mathematics, 09.04.2020 18:34


Social Studies, 09.04.2020 18:34





History, 09.04.2020 18:34


Mathematics, 09.04.2020 18:34

Mathematics, 09.04.2020 18:34

Mathematics, 09.04.2020 18:34





English, 09.04.2020 18:34

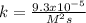
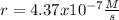
![r=k[NO]^2[O_2]](/tpl/images/0747/3983/b9fab.png)
![k=\frac{r}{[NO]^2[O_2]}\\\\k=\frac{0.00022022M/s}{(0.8M)^2(3.7M)} \\\\k=\frac{9.3x10^{-5}}{M^2s}](/tpl/images/0747/3983/c4bb6.png)