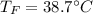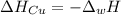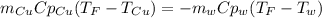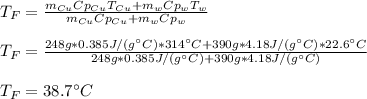A 248-g piece of copper initially at 314 °C is dropped into 390 mL of water initially at 22.6 °C. Assuming that all heat transfer occurs between the copper and the water, calculate the final temperature. The specific heat of copper (0.385 J/goC) and water (4.18 J/goC) and density of water (1.00 g/mL) will be needed.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
A 248-g piece of copper initially at 314 °C is dropped into 390 mL of water initially at 22.6 °C. As...
Questions

Mathematics, 30.10.2020 19:30

Computers and Technology, 30.10.2020 19:30


English, 30.10.2020 19:30

History, 30.10.2020 19:30





Chemistry, 30.10.2020 19:30

English, 30.10.2020 19:30


Health, 30.10.2020 19:30

Social Studies, 30.10.2020 19:30

Mathematics, 30.10.2020 19:30





Mathematics, 30.10.2020 19:30