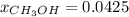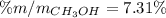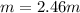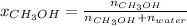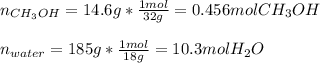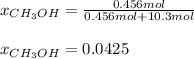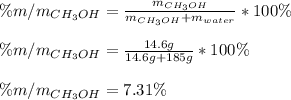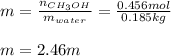Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2


Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
A solution is made containing 14.6g of CH3OH in 185g H2O.1. Calculate the mole fraction of CH3OH.2....
Questions

Mathematics, 25.02.2020 16:10

Mathematics, 25.02.2020 16:10

Health, 25.02.2020 16:16


Computers and Technology, 25.02.2020 16:16

History, 25.02.2020 16:16


Biology, 25.02.2020 16:16



Biology, 25.02.2020 16:16


Mathematics, 25.02.2020 16:16

History, 25.02.2020 16:16