
Chemistry, 09.09.2020 23:01 j1theking18
What are the final hydrogen ion concentration and pH of a solution obtained by mixing 400mL of 0.2M NaOH with 150mL of 0.1M H3PO4?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

You know the right answer?
What are the final hydrogen ion concentration and pH of a solution obtained by mixing 400mL of 0.2M...
Questions


Computers and Technology, 14.07.2019 22:00

Chemistry, 14.07.2019 22:00



Chemistry, 14.07.2019 22:00

Mathematics, 14.07.2019 22:00


Mathematics, 14.07.2019 22:00


English, 14.07.2019 22:00

Mathematics, 14.07.2019 22:00





History, 14.07.2019 22:00


English, 14.07.2019 22:00

Chemistry, 14.07.2019 22:00

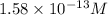
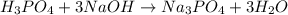
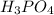 reacts with 3 mole NaOH.
reacts with 3 mole NaOH.
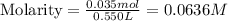
![[OH^-]](/tpl/images/0748/0545/b2910.png) = 0.0636 M
= 0.0636 M![pOH=-\log [OH^-]](/tpl/images/0748/0545/1fac1.png)
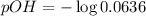
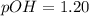
![pH=-\log [H^+]](/tpl/images/0748/0545/37e81.png)
![12.8=-\log [H^+]](/tpl/images/0748/0545/12b90.png)
![[H^+]=1.58\times 10^{-13}M](/tpl/images/0748/0545/66dc4.png)


