
Chemistry, 19.09.2020 01:01 emileehogan
Hydrogen gas, H2, reacts explosively with gaseous chlorine, Cl2, to form hydrogen chloride, HCl(g). What is the enthalpy change for the reaction of 2 mole of H2(g) with 2 mole of Cl2(g) if both the reactants and products are at standard state conditions? The standard enthalpy of formation of HCl(g) is −92.3 kJ/mol. H2(g)+Cl2(g)→2HCl(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
Hydrogen gas, H2, reacts explosively with gaseous chlorine, Cl2, to form hydrogen chloride, HCl(g)....
Questions

Chemistry, 18.09.2019 14:50

History, 18.09.2019 14:50






Mathematics, 18.09.2019 14:50


History, 18.09.2019 14:50

Mathematics, 18.09.2019 14:50


Biology, 18.09.2019 14:50


Social Studies, 18.09.2019 14:50

Health, 18.09.2019 14:50

Mathematics, 18.09.2019 14:50

Mathematics, 18.09.2019 14:50


History, 18.09.2019 14:50

 , reacts explosively with gaseous chlorine,
, reacts explosively with gaseous chlorine,  , to form hydrogen chloride, HCl(g).
, to form hydrogen chloride, HCl(g).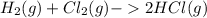
 with 2 mole of
with 2 mole of  if both the reactants and products are at standard state conditions .
if both the reactants and products are at standard state conditions .


