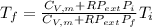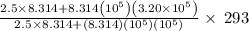An automobile tire contains air at 320.×103 Pa at 20.0 ◦C. The stem valve is removed and the air is allowed to expand adiabatically against the constant external pressure of 100.×103 Pa until P = Pexternal. Assume the air is an ideal gas with C¯ V = 5/2 R (diatomic). Calculate the final temperature.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

You know the right answer?
An automobile tire contains air at 320.×103 Pa at 20.0 ◦C. The stem valve is removed and the air is...
Questions

Mathematics, 20.11.2020 18:40


Mathematics, 20.11.2020 18:40

Chemistry, 20.11.2020 18:40


Computers and Technology, 20.11.2020 18:40

World Languages, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40

English, 20.11.2020 18:40



Health, 20.11.2020 18:40


Mathematics, 20.11.2020 18:40


Biology, 20.11.2020 18:40