
Chemistry, 19.09.2020 01:01 BitFox1684
NEED HELP PLEASE ANSWER ALL!
1) In a solid metal sample, how are valence electrons distributed?
a) Valence electrons are shared between neighboring metal atoms to form covalent bonds.
b) Valence electrons are shared among many metal ions.
c) Valence electrons are unequally distributed, giving some metal ions positive charge and some metal ions negative charge.
d) Valence electrons are localized to each metal atom, giving it a neutral charge.
2) The overall process of photosynthesis has the balanced chemical equation 6CO2 + 6H2O → C6H12O6 + 6O2. What does this tell you about the relative amounts of carbon dioxide and water consumed in this process?
a) For every gram of carbon dioxide consumed, one gram of water is consumed.
b) For every six moles of carbon dioxide consumed, one mole of water is consumed.
c) For every six grams of carbon dioxide consumed, one gram of water is consumed.
d) For every mole of carbon dioxide consumed, one mole of water is consumed.
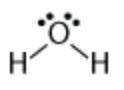
3) In this molecule, what's the formal charge on the central O atom? IMAGE ABOVE!
a) 0 b) + 1 c) - 2 d) - 1
4) The possible products of a double displacement reaction in aqueous solution are (NH4)2S and NaNO3. Which of these possible products will form as a solid in this reaction?
a) (NH4)2S
b) NaNO3
c) Neither compound will form as a solid.
d) Both compounds will form as a solid.
5) Which process is used to produce gases from solutions of salts dissolved in water or another liquid?
a) Electrolysis
b) Polar covalent bonding
c) Metallic bonding
d) Ionic bonding
6) When NaCl is dissolved in water, it separates into Na+ and Cl– ions, which are also dissolved in water. How would the phase of the NaCl, the Na+, and the Cl– be written in the reaction equation?
a) (g) b) (s) c) (l) d) (aq)
7) In general, which of the following statements about electron-pair geometries is true?
a) They maximize the space between valence electrons to minimize the repulsion between them.
b) They maximize the polarity of valence electrons to minimize the attraction between them.
c) They maximize the polarity of valence electrons to minimize the repulsion between them.
d) They maximize the space between valence electrons to minimize the attraction between them.
8) Iron oxide reacts with carbon monoxide to produce iron and carbon dioxide, with the balanced chemical equation Fe2O3 + 3CO → 2Fe + 3CO2. What does this tell you about the relative amounts of iron oxide and iron consumed and produced in this equation?
a) For every two grams of iron oxide consumed, one gram of iron is produced.
b) For every gram of iron oxide consumed, two grams of iron are produced.
c) For every mole of iron oxide consumed, two moles of iron are produced.
d) For every two moles of iron oxide consumed, one mole of iron is produced.
9) A decomposition reaction causes a chemical to break apart into two products. You measure the rate of the reaction and find that it proceeds quickly at first, but slows down over time. How would you explain this result?
a) The concentration of the starting material decreases over time.
b) The concentrations of the products decrease over time.
c) The concentration of the starting material increases over time.
d) The rate of decomposition reactions doesn't change over time; there must be a measurement error.
10) How does the average reaction rate differ from an instantaneous reaction rate?
a) The average reaction rate is how quickly the reaction proceeds at a specific time. An instantaneous reaction rate is how quickly the reaction proceeds over time.
b) The average reaction rate is how quickly the reaction proceeds over time considering the reactants. An instantaneous reaction rate is how quickly the reaction proceeds at a specific time considering the products.
c) The average reaction rate is how quickly the reaction proceeds over time. An instantaneous reaction rate is how quickly the reaction proceeds at a specific time.
d) The average reaction rate is how quickly the reaction proceeds over time. An instantaneous reaction rate is how quickly the reaction proceeds compared to another reaction.
11) In a certain chemical reaction, 2 solid Mg atoms bond with O2 gas to produce solid MgO. Which of the following reaction equations correctly describes this reaction?
a) 2Mg (s) + O2 (g) → 2MgO (s)
b) 2Mg (s) + O2 (g) → MgO (l)
c) Mg (s) + 2O2 (g) → MgO (s)
d) Mg (g) + O2 (s) → 2MgO (g)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 06:30
How can the number of core electrons be determined from the periodic table
Answers: 1

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1
You know the right answer?
NEED HELP PLEASE ANSWER ALL!
1) In a solid metal sample, how are valence electrons distributed?
Questions

Mathematics, 30.11.2020 20:30

Mathematics, 30.11.2020 20:30

Social Studies, 30.11.2020 20:30

Physics, 30.11.2020 20:30

Biology, 30.11.2020 20:30

Mathematics, 30.11.2020 20:30

Mathematics, 30.11.2020 20:30

Chemistry, 30.11.2020 20:30

English, 30.11.2020 20:30




Mathematics, 30.11.2020 20:30







English, 30.11.2020 20:30



