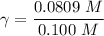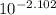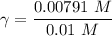Chemistry, 20.09.2020 18:01 jamesgraham577
A. The measured pH of a 0.100 M HCl solution at 25 degrees Celsius is 1.092. From this information, calculate the activity coefficient of H+.B. The measured pH of a solution of 0.010 HCl and 0.090 KCl at 25 degree Celsius is 2.102. Calculate the activity coefficient of H+ in this solution. C. Why does the pH change in part B relative to that in part A?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1


Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
A. The measured pH of a 0.100 M HCl solution at 25 degrees Celsius is 1.092. From this information,...
Questions


Mathematics, 28.09.2019 13:10

Mathematics, 28.09.2019 13:10


History, 28.09.2019 13:10


Mathematics, 28.09.2019 13:10


History, 28.09.2019 13:10

Mathematics, 28.09.2019 13:10

Mathematics, 28.09.2019 13:10






Mathematics, 28.09.2019 13:10

Mathematics, 28.09.2019 13:10

Mathematics, 28.09.2019 13:10

Chemistry, 28.09.2019 13:10

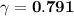
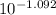
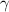 × C
× C![\gamma = \dfrac{[a]}{C}](/tpl/images/0773/2720/33e10.png)