
Chemistry, 20.09.2020 05:01 chewygamerz
HELP!!
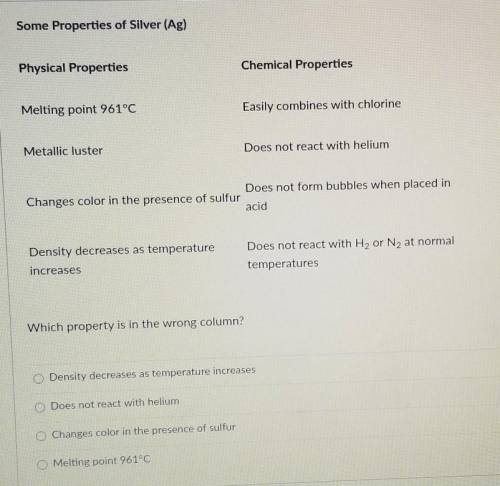
Cade missed chemistry class because he was ill. He borrowed some notes from his lab partner to review what he missed. As Cade was copying the notes, he noticed a mistake in his partner's table, which is shown below. Some Properties of Silver (Ag) Chemical Properties Physical Properties Easily combines with chlorine Melting point 961°C Does not react with helium Metallic luster Does not form bubbles when placed in Changes color in the presence of sulfur acid Density decreases as temperature increases Does not react with H2 or N2 at normal temperatures
Which property is in the wrong column?

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
HELP!!
Cade missed chemistry class because he was ill. He borrowed some notes from his lab partner...
Questions


Health, 08.07.2019 08:00

Mathematics, 08.07.2019 08:00

Mathematics, 08.07.2019 08:00


English, 08.07.2019 08:00




Mathematics, 08.07.2019 08:00


Mathematics, 08.07.2019 08:00


History, 08.07.2019 08:00


History, 08.07.2019 08:00

Social Studies, 08.07.2019 08:00





